Table of Contents
Properties of Ammonia:
Physical Properties of Ammonia:
- Ammonia is a colourless gas with a characteristic pungent smell. It has a bitter burning taste.
- It is lighter than air.
- It is highly soluble in water and the solution is basic in nature. One volume of water dissolves 1300 volumes of ammonia at 0°C and 760 mm. The extreme solubility of ammonia in water can be demonstrated by the Fountain Experiment as in case of HCl gas.
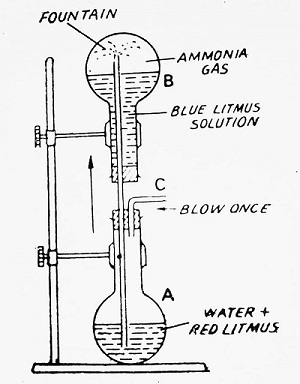
Fountain Experiment- Fill a dry flask B with dry ammonia gas. In flask A, take some water to which some red litmus has been added. Fit up the apparatus as indicated in the figure. On blowing at C, some drops of water are introduced into flask B. This small quantity of water dissolves a large quantity of gas and pressure inside the flask B is greatly reduced. Due to this, water from flask A rushes up into flask B in the form of a beautiful blue fountain. This demonstrates that an aqueous solution of ammonia is basic (ammonia turns red litmus blue in nature).
- It can be liquefied to a colourless liquid (boiling point, -33.4°C) and frozen to a snowy white solid (melting point, -78°C).
- Its concentrated solution is corrosive to the skin.
Chemical Properties of Ammonia:
(1) Basic Nature- Ammonia is basic in nature. It turns moist red litmus paper blue, turmeric paper brown and phenolphthalein pink. It dissolves in water to give ammonium hydroxide.
| NH3 + H2O ————-> NH4OH |
On interaction with acids like HCl etc., it forms the corresponding salt.
| NH3 + HCl ————-> NH4Cl |
(2) Combustion- Ammonia is neither combustible nor a supporter of combustion.
(3) Oxidation- (a) Under suitable conditions, ammonia burns in an atmosphere of oxygen with a greenish-yellow flame giving rise to the formation of nitrogen and water.
| 4NH3 + 3O2 ————-> 2N2 + 6H2O |
(b) On passing over heated copper oxide, ammonia is oxidized to form nitrogen.
| 2NH3 + 3CuO ————-> N2 + 3Cu + 3H2O |
(c) Ammonia mixed with air or oxygen in the presence of platinum gauze as a catalyst at 800-850°C, is oxidized to nitric oxide (NO).
| 4NH3 + 5O2 ————-> 4NO + 6H2O |
(4) Dissociation- Ammonia dissociates into nitrogen and hydrogen when it is heated to above 1100°C or subjected to electric sparks.
| 2NH3 ————-> N2 + 3H2 |
(5) Action of Halogens- Ammonia is oxidized by halogens.
Action of chlorine- When ammonia is in excess, nitrogen is evolved.
| 8NH3 + 3Cl2 ————-> N2 + 6NH4Cl |
When chlorine is in excess, nitrogen trichloride (a highly explosive compound) is formed.
| NH3 + 3Cl2 ————-> NCl3 + 3HCl |
Bromine also behaves in the same way towards ammonia.
(6) Action of Metals- Ammonia reacts with metals at elevated temperatures giving rise to the formation of either amides or nitrides. For example-
| 2NH3 + 2Na ————-> 2NaNH2 (Sodamide) + H2 2NH3 + 3Mg ————-> Mg3N2 (Magnesium nitride) + 3H2 2NH3 + 2Al ————-> 2AlN (Aluminium nitride) + 3H2 |
(7) Reactions of Ammonium Hydroxide- Ammonia dissolves in water to give ammonium hydroxide. The aqueous solution of ammonia is basic in nature.
| NH3 + H2O ————-> NH4OH |
(a) Action of acids- Ammonium hydroxide reacts with acids to form the corresponding ammonium salts and water. For example-
| NH4OH + HNO3 ————-> NH4NO3 (Ammonium nitrate) + H2O 2NH4OH + H2SO4 ————-> (NH4)2SO4 (Ammonium sulfate) + 2H2O |
(b) Formation of complex compounds- On treatment with certain compounds, ammonia gives rise to the formation of soluble complexes. For instance,
Action of silver chloride- Silver chloride (AgCl) dissolves in excess of NH4OH to give soluble diammine silver (I) chloride complex.
| AgCl + 2NH4OH ————-> [Ag(NH3)2]Cl + 2H2O |
Action of copper sulfate- Copper sulfate reacts with NH4OH to give a blue precipitate of copper hydroxide.
| CuSO4 + 2NH4OH ————-> Cu(OH)2 + (NH4)2SO4 |
If an excess of NH4OH is added, the light blue precipitate of copper hydroxide dissolves to give a deep blue solution of cuprammonium hydroxide complex.
| Cu(OH)2 + 4NH4OH ————-> [Cu(NH3)4] (OH)2 + 4H2O |
(c) Precipitation of hydroxides- The addition of ammonium hydroxide solution to an aqueous solution of salts of certain metals like Fe, Al etc., results in the formation of precipitates. For example-
| Al2(SO4)3 + 6NH4OH ————-> 2Al(OH)3 + 3(NH4)2SO4 |
Uses of Ammonia:
Ammonia is used-
- In the manufacture of nitric acid by Ostwald’s process.
- In the manufacture of chemical fertilizers such as ammonium phosphate, ammonium sulfate and ammonium nitrate.
- As a highly useful refrigerant in mechanical refrigerators, ice making establishments and cold storage plants.
- In the manufacture of sodium carbonate by Solvay’s process.
- As a laboratory reagent for the precipitation of metal hydroxides.
- In the manufacture of many ammonium compounds such as NH4OH (used as cleansing agent), NH4Cl (used as an electrolyte in dry cells) and NH4NO3 (used in making explosives).








Comments (No)