Scale of Temperature:
The concept of thermal equilibrium helps to find bodies that are at the same temperature and also to know which is at a higher temperature with respect to the other. Next, we wish to define a scale of temperature so that we can assign a numerical value to the temperature of the body. This can be done by choosing a measurable property of a substance that monotonically changes with a change in temperature. The temperature can then be related to the value of this quantity. For example, consider an amount of mercury contained in a glass bulb having a long capillary attached to it. The length of the mercury column in the capillary changes when the temperature changes. Corresponding to different lengths, we can assign different numerical values to the temperature. Let us see how it is done.
The method which is in practice for a long time is to choose two fixed points of temperature which can be easily reproduced in the laboratory. Such convenient points are ice-point (the temperature of pure melting ice at 1 atmospheric pressure) and steam-point (the temperature of pure boiling water at 1 atmospheric pressure). These temperatures are given arbitrary numerical values and the interval between them is divided into an arbitrary number of equal parts. Each part is called a degree which is the unit of temperature. For example, let l1 and l2 be the lengths of the mercury column in the capillary tube attached to the bulb containing mercury when the bulb is kept in the melting ice (ice-point) and boiling water (steam-point), respectively. Obviously, a change of one degree in temperature will change the length of the mercury column by l2-l1/tst-tice provided we assume a linear relationship (t = gl + b) between length (l) and temperature (t). Any unknown temperature can now be determined by noting the length of the mercury column in the capillary when the bulb is kept at the unknown temperature.
The ice-point and the steam-point have been given different numerical values in different scales of temperature. These scales are-
| Ice-point | Steam-point | |
| Celsius scale (formerly called Centigrade scale) | 0°C | 100°C |
| Fahrenheit scale | 32°F | 212°F |
| Kelvin scale | 273.15 K | 373.15 k |
Conversion of temperature from one scale to another can be made by using the identity (relating the three scales)-
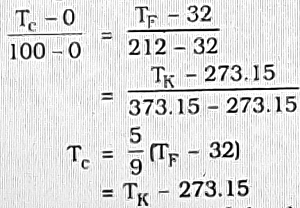
It is to be noted that the size of the degree is same on Celsius and Kelvin scale or absolute scale. It is called absolute scale because it does not depend on the property of the working substance.








Comments (No)