Table of Contents
Electroplating:
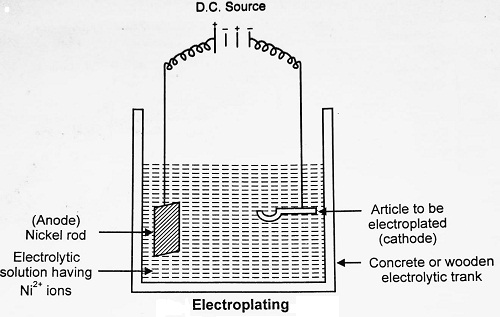
In this process, the coating metal is deposited on the base metal by passing a direct current through an electrolytic solution containing soluble salt of the coating metal. The metal to be plated (base metal) is made the cathode. The anode is usually either the metal to be deposited or an inert material of good conductivity like graphite.
For example Nickel Plating. In this case, the article to be electroplated is first cleaned and is then made cathode in an electrolytic bath. The electrolyte is a solution of salt containing Ni2+ ions having good solubility and conductivity. The solution usually taken in this case is of double salt [NiSO4.(NH4)SO4]. The anode is pure Ni or graphite.
On passing direct current (D.C.), Ni2+ ion present in solution move towards the cathode and get deposited on it in the form of a uniform metallic layer. An equivalent amount of Nickel is dissolved from the Nickel anode into the solution.
Electroless Plating:
In this method, a noble metal (from its salt solution) is deposited on a less noble metal without using electrical energy in the presence of a suitable reducing agent. The reducing agent causes the reduction of metallic ions (of more active metal) to metal which gets deposited over less noble metal i.e.
| Metal Ions ——–Reducing Agent———-> Metal (Deposited over less active Metal) |
The Technique- The electroless plating involves-
(1) Activation of the surface of a metal to be plated. This is done by etching, treating with chemicals etc.
(2) Plating bath. It is composed of a soluble salt of metal, reducing agent, complexing agent, stabilizer and buffer solution. Examples are Electroless Ni plating, Electroless Cu plating.
Advantages Over Electroplating:
(1) No electrical energy is required.
(2) Articles of irregular surfaces can be uniformly plated.
(3) Better throwing power.









Comments (No)