Phenomenon of Electrolysis:
In electrochemical cells, chemical energy is converted into electrical energy. In the reverse case, the passage of electricity through electrolytes under suitable conditions may cause chemical changes. The process of chemical decomposition of an electrolyte by the passage of electricity through it in the molten state or dissolved state is known as Electrolysis.
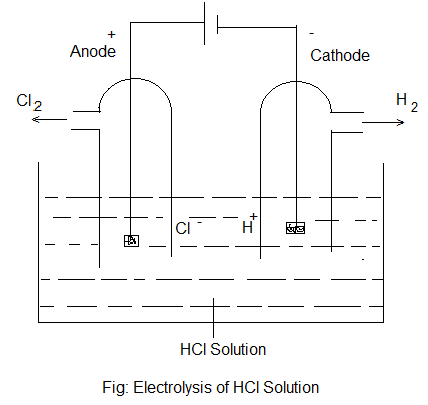
In this process, two metal rods (known as electrodes) are placed in an electrolyte (in solution or in molten state) contained in a vessel made of a non-conducting material. One electrode is connected to the positive terminal of the battery and is called “Anode” or Positive electrode while the other is connected to the negative terminal and is called “Cathode” or Negative electrodes. On electrolysis, the negatively and positively charged ions of the electrolyte move towards oppositely charged electrode i.e. cations move towards cathode and anions towards the anode. At the electrodes, the ions give primary and secondary products. For example- in the electrolysis of an aqueous solution of HCl, the reaction taking place are-
| Ionisation: HCl (aq) ⇌ H+ (aq) + Cl– (aq) |
| At Cathode: H+ (aq) + e– ————> H (Primary Product) H + H ————> H2 (Secondary Product) |
| At Anode: Cl– (aq) – e– ————> Cl (Primary Product) Cl + Cl ————-> Cl2 (Secondary Product) |
The reactions taking place in the electrolysis of molten NaCl are-
| At Cathode: Na+ + e– ————> Na |
| At Anode: Cl– – e– ———> Cl Cl + Cl ——-> Cl2 (g) |
These reactions indicate that passage of one mole of electrons gives one mole of sodium and two moles of electrons produce 1 mole of Cl2.
Charge on one mole of electrons = 1.602 x 10-19 coulombs X 6.023 x 1023 = 96488 coulombs ≃ 96500 coulombs = 1 Farady or 1 F.
Therefore, the charge on ‘n’ moles of electrons = nF.
According to this relation-
Production of 1 mole of Na from Na+ ions require 1 mole of electrons = 1 F = 96500 coulombs of charge.
Production of 1 mole of Cl2 from Cl– ions require 2 mole of electrons = 2 F = 2 x 96500 coulombs of charge.
Production of 1 mole of Al from Al3+ ions require 3 moles of electrons = 3 F = 3 x 96500 coulombs of charge.
Now, 1 Coulomb = 1 Ampere x 1 Second
Thus, it is possible to calculate the amount of substance deposited or evolved (if gas) when a definite amount of current is passed for a definite period of time.








Comments (No)