Pressure of Atmosphere:
Our earth is surrounded by air up to a considerable height. This envelope of air surrounding the Earth is called the atmosphere. We live at the bottom of an ocean of air- the atmosphere.
The atmosphere is composed of several gases, chiefly nitrogen, and oxygen. The density of air is maximum at the surface of the earth and it goes on decreasing with height. It is not possible to give the exact height of the atmosphere because the atmosphere does not abruptly end at any height- it gradually becomes thinner and ultimately fades out.
The composition of the atmosphere is not uniform, however, near the surface of the earth, it consists of about 78.1% of nitrogen, 20.9% of oxygen, about 1% of water vapor, 0.03% of carbon dioxide, and traces of argon, hydrogen, neon, krypton, xenon, and ozone.
Since air has weight, a column of air exerts pressure as does a column of liquid. The following experiments clearly demonstrate the existence of atmospheric pressure.
(1) Glass Tumbler and Cardboard Experiment- Take a glass tumbler and fill it completely with water. Cover it with a thin piece of cardboard or a postcard, taking care to see that it is in close contact with the surface of the water and no air is trapped between the water’s surface and the cardboard. Then keeping the cardboard in position by one hand, invert the tumbler as shown below.
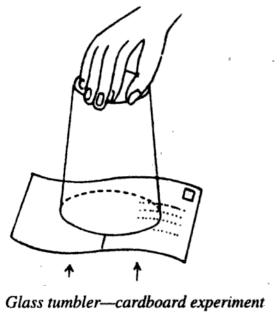
When the hand holding the cardboard is removed, the water does not fall. Why? It is because a force due to the atmospheric pressure acts on the cardboard which pushes it upwards.
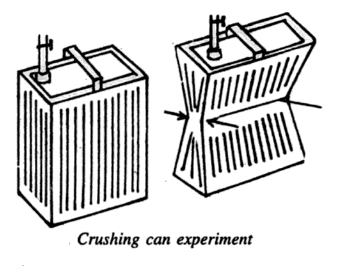
(2) Crushing Can Experiment- Take a tin can and partly fill it with water. Heat it till most of the air has been driven out of the can by the steam produced. Now, close the can with its stopper and allow it to cool.
As the can cools down, the steam present in the can condenses producing water and water vapor at low pressure. Now the atmospheric pressure acting from outside cannot be balanced by the inside pressure, as almost all of the air was driven out. The can, therefore, crushes under the excessive pressure of the atmosphere as shown below.
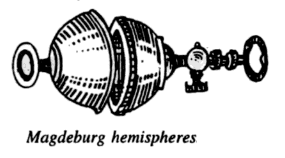
(3) Magdeburg Hemisphere Experiment- This experiment was originally done in 1654 by German Scientist Otto Von Guericke. He fitted two hemispheres together and then extracted the air from inside with the help of an air pump designed by him. Before the extraction of air, it was quite easy to pull the hemispheres apart, however, after the extraction of air it was not possible to do so. Even two teams of eight horses could not pull them apart.
On extraction of air from the inside of the hemispheres, the only force acting was due to atmospheric pressure. The force due to the atmosphere pressed the two spheres so hard that even two teams of horses could not separate them.







Comments (No)