Rutherford Model:
Atoms are not like solid balls. After a series of experiments, Rutherford discovered that inside an atom there exists a small piece of matter called a nucleus where practically the whole mass of the atom is concentrated as shown below. The nucleus is positively charged and negatively charged electrons revolve around the nucleus.
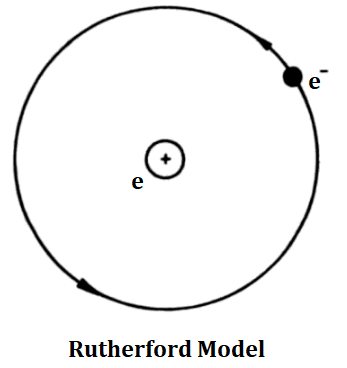
Let us consider the lightest of all elements, hydrogen. It has one electron. The charge on the nucleus is +e and that on the electron is -e. Suppose the electron is revolving in a circular orbit of radius r with a speed ν. The centripetal force mν2/r is provided by Coulomb’s attraction e2/4πε0r2 between the electron and the nucleus. Thus,
| mν2/r = e2/4πε0r2 ……….(i) |
The total energy E of the electron is the sum of its kinetic energy K and potential energy U, i.e.,
| E = K + U ……….(ii) |
The kinetic energy of an electron is (1/2)mν2, therefore, using equation (i), we have
| K = (1/2)mν2 = (1/2) (e2/4πε0r) ……….(iii) |
The potential at a distance r from a charge e is known to be e/4πε0r, hence the potential energy of an electron that carries a charge -e is given by-
| U = (e/4πε0r) x -e = -e2/4πε0r ……….(iv) |
From (ii), (iii), and (iv), we have
| E = (-1/2) (e2/4πε0r) ……….(v) |
The energy of an electron is negative. The significance of total energy being negative is that the system is a closed one i.e., the electron is bound to the attracting force of the nucleus and it cannot leave the atom until some energy is supplied to it. It should be noted that the nucleus being at rest, the above relation also gives the total energy of the atom.
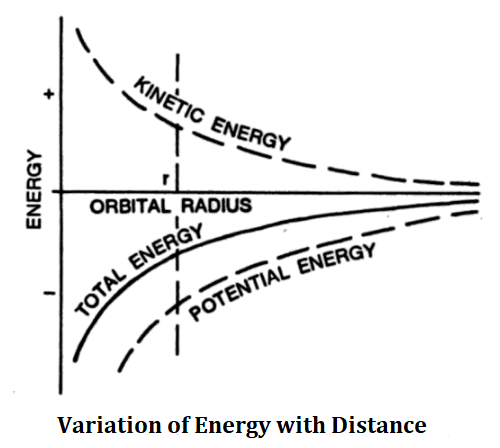
The variation of kinetic energy K, potential energy U, and total energy E with distance r from the nucleus has been shown below.
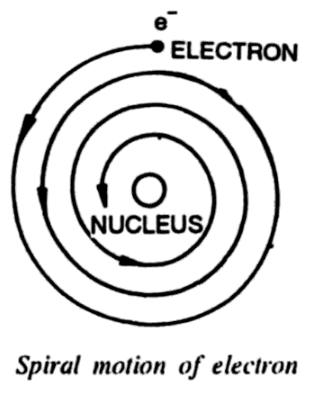
An electron revolving around the nucleus has an acceleration directed towards the nucleus. Since it is known from the classical electromagnetic theory that an accelerating electron must radiate electromagnetic radiations, the revolving electron in the Rutherford model must also radiate energy.
The electromagnetic energy so radiated must have a frequency equal to the frequency of revolution of the electron. But if a revolving electron radiates energy, the total energy of the system must decrease. From the above figure, it is clear that if energy decreases, the electron comes closer to the nucleus with increasing speed until it hits the nucleus. Further, such a spiral motion of an electron should radiate electromagnetic radiations of continuous frequencies hence the spectrum of hydrogen should be a continuous spectrum, which is not the fact. Hydrogen emits a line spectrum.







Comments (No)