Relative Permittivity or Dielectric Constant:
If the electric charges are immersed in some medium, the presence of the medium does affect the force between the charges. The Coulomb’s relation as it is given by the equation below is then not applicable.

When the charges are immersed in a real medium, the effect of induced charges must be taken into account and thus the problem becomes quite complicated. However, under certain situations, it is possible to say that when the charges are immersed in a real medium, the force between the charges Q1 and Q2 is given by a similar relation.

Where ε is called the absolute permittivity of the medium. From practical considerations, it is better to introduce the term relative permittivity εr defined by the relation-
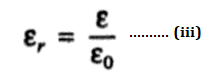
As is obvious εr is a dimensionless number and has no unit. For gases, εr is slightly more than 1 (For air εr = 1.006). For most substances, it has a value of 1 to 10. Till recently, we used to call this ratio the dielectric constant and used to represent the dielectric constant by the symbol K. Thus εr and K have the same meaning and in terms of dielectric constant, relation (ii) can be written as-

From relations (i) and (iv), we find that the force between charges is reduced by a factor K as soon as the charges are immersed in a real medium instead of a vacuum. It should be noted that the above relation is approximate, applicable under certain situations only (medium around charges is homogeneous, isotropic fluid extending up to infinity), hence roughly dielectric constant can be defined as the ratio of the force between two point charges when placed at a certain distance apart in air to the force between the same two charges when placed the same distance apart in the given medium.








Comments (No)