Table of Contents
Thermotropic Liquid Crystals:
In thermotropic liquid crystals the molecular ordering changes with temperature. The molecules of liquid crystals have rod-like shapes, with a central rigid portion and flexible ends. According to different types of molecular ordering, thermotropic crystals are classified as nematics, cholesterics, and smectics.
Nematic Liquid Crystals:
Nematic liquid crystals have rod-like molecules and the molecules naturally tend to orient their long axes along a direction called the director axis, as shown below. The entire nematic liquid crystal consists of small regions, each having its molecules aligned parallel to a unique axis, but this axis generally varies in direction in different regions of the crystal. This feature accounts for a high optical inhomogeneity of the crystal, which appears opaque in the transmitted and reflected light.
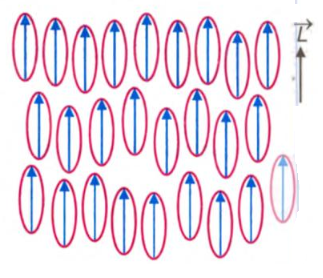
An externally applied field orients the molecules of all regions in the same direction, and the liquid crystals become transparent. Placing a thin layer of the nematic liquid between two plate electrodes enables aligning the molecules both parallel, figure (a) and normal, figure (b), to the planes of the conducting plates. The first type of alignment is said to be planar and the second hemotropic. The latter structure, unlike the former, has no effect on the polarization of light transmitted through the cell at a right angle to the layer of the liquid crystal. Special treatment of the surfaces of plate electrodes or requisite chemical aligning agents introduced in the liquid gives the desired orientation of molecules. An electric field produced between the plates and various other factors can change this orientation and this alters the optical properties of the liquid crystal.
Pure nematic crystals are insulators. To make them conducting, they are to be doped with ionic impurities.
Cholesteric Liquid Crystals:
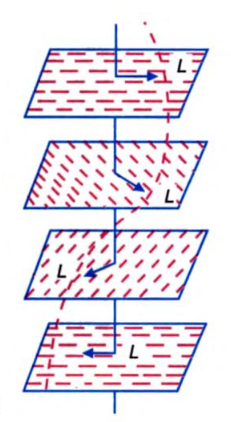
The structure of a cholesteric liquid crystal is shown below. A cholesteric liquid crystal has its structure twisted about the helical axis lying perpendicular to the orientation of molecules. The vector L is called the director. In going from one plane to the other, the vector L turns by a certain angle and its end describes a helix with a pitch (structure period) of about 0.2 to 20 μm. The periodicity of the structure along the helical axis results in Bragg’s reflection of light at a wavelength equal to the pitch divided by the refractive index of the liquid crystal. A liquid crystal has high optical activity. So the cholesteric fluid causes the plane of polarization of light to turn through extremely large angles, of the order of 6,000 to 7,000° / mm. But in such an optically active crystal as quartz, the angle of turn of the light polarization plane is 15.5° / mm.
An external field applied to the cholesteric fluid can change the pitch of the helix, turn the helical axis, and convert the fluid to the nematic type. Therefore, the transmission of light by the system consisting of the layer of the cholesteric liquid placed between two polarizers will change with the applied potential difference. This is the principle that underlies the operation of various displays, and liquid crystal digital indicators applied in some types of electronic watches, clocks, microcalculators, etc.
Smectic Liquid Crystals:
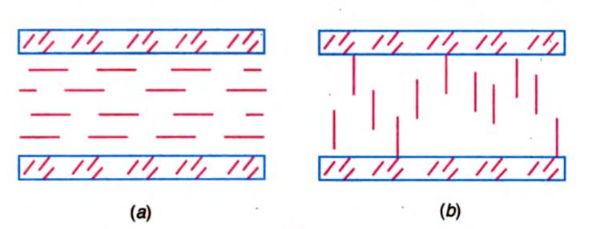
The most ordered smectic crystals have a layer structure. Molecules align themselves in parallel layers gliding one relative to the other, thereby promoting fluidity. The long axis of molecules lines up either perpendicular to the planes of layers in Figure (a) or forms a certain angle Φ with the normal to these planes in Figure (b). The value of this angle Φ depends on the temperature of the liquid crystal and can be altered strongly by an externally applied field.










Comments (No)