Sodium Vapour Lamp:
Construction: It consists of two glass tubes, an outer glass tube and an inner glass tube. The inner glass tube contains two electrodes. Sodium along with a small quantity of neon or argon gas is filled in an inner tube to make discharge self-starting. Sodium vapour is chemically very active. The glass of the tube is made up of suitable material to resist this action.
To maintain the correct temperature in the discharge, it is placed in an evacuated outer tube. The outer tube reduces the heat loss. The transformer included in the circuit heats the cathode while the choke stabilizes the discharge.
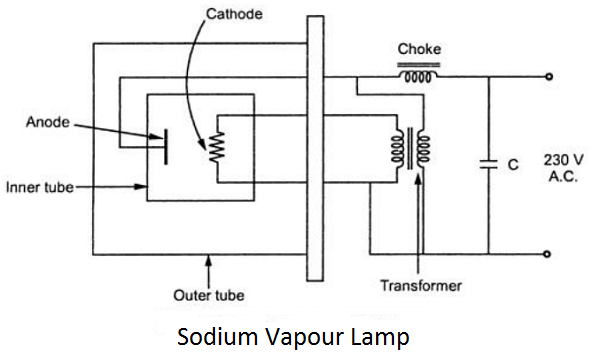
Working: When the lamp is switched on, the discharge is first established through the neon or argon gas, this gives out a reddish colour. After some time heat is developed due to this discharge which vaporizes sodium vapour. In this way, the lamp starts its normal operation giving yellow colour. Capacitor C is connected to have a better power factor. The operating temperature of this lamp is about 300. These lamps are commonly used for illumination of roads, good yards, airports etc.
Advantages: (i) Its efficiency is higher than that of the filament lamps.
(ii) It has a long life.
Disadvantages: (i) The bright yellow colour obtained is not suitable for indoor lighting. So it is not useful in houses.
(ii) For the necessary output, long tubes are required.
(iii) For giving full output, some time (about 10 minutes) is required.









Comments (No)