Table of Contents
Abnormal Molecular Mass:
When the molecular mass of a substance determined by studying any of the colligative properties comes out to be different from theoretically expected values the substance is said to show abnormal molecular mass. For example- the molecular mass of benzoic acid (C6H5COOH) is theoretically expected to be 122 whereas the molecular mass of benzoic acid determined experimentally by use of any colligative property comes out to about 240.
Such a molecular mass is called abnormal molecular mass. The abnormal molecular mass is attributed to any one of the following reasons-
- Association of solute molecules in a solution.
- Dissociation of solute molecules in a solution.
Association of Solute in Solution:
When the solute, whose molecular mass is to be determined, undergoes association in solution, the number of moles of such substance decrease. Since the colligative properties are inversely proportional to molecular mass and are directly proportional to the molar concentration of solute; for the solutes which undergoes association, show a decrease in colligative properties and thus their molecular mass will show an unexpected increase in molecular mass. Example- Benzoic acid exists as dimers due to H-bonding in solution due to association (as shown below) and hence its molecular mass will show an increase i.e.,
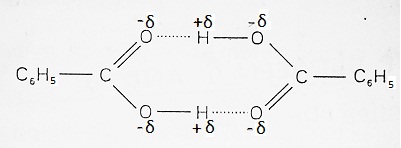
Dissociation of Solute in Solution:
When an ionic non-volatile solute is added to a solvent to get the molecular mass of solute, such solute undergoes ionic dissociation, For Example- KCl.
| KCl = K+ + Cl– |
In such a case, the number of ions increases, thereby the colligative property also increases. This increase in colligative property decreases the molecular mass of solute.
Hence, the molecular mass show abnormality.







Comments (No)