Avogadro Law:
Avogadro, an Italian scientist, suggested that the smallest unit of matter capable of independent existence in a gas is a molecule and the volume of a gas is related to the number of molecules present in it. According to him, equal volumes of all gases, under similar conditions of temperature and pressure, contain an equal number of molecules. This generalisation is called Avogadro’s law. This law has been useful in the development of chemistry. For example-
(I) It has been helpful in the calculation of atomicity of elementary gases.
(Atomicity of an element is defined as the number of atoms contained in its molecule).
For illustration, consider the reaction between Hydrogen and chlorine to yield gaseous Hydrogen chloride.
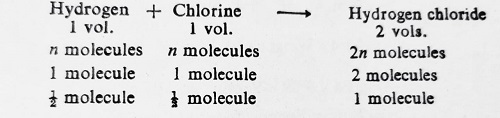
According to this reaction, one molecule of Hydrogen chloride contains 1/2 molecule of Hydrogen. Since Hydrogen chloride forms only one series of salts, i.e., chlorides, it has, therefore, only one atom of Hydrogen. This indicates that 1/2 molecule of Hydrogen = 1 atom of Hydrogen. Hence, 1 molecule of Hydrogen contains two atoms. In other words, the atomicity of Hydrogen is 2.
(II) It has helped in deriving the relationship between molecular weight and vapour density of gases.
| Molecular Weight = 2 x Vapour Density Vapour Density = Weight of a certain volume of gas or vapour/Weight of same volume of hydrogen at same temperature and pressure |
Applying Avogadro’s law, we get-
| Vapour Density = Weight of n molecules of gas or vapour / Weight of n molecules of hydrogen = Weight of 1 molecule of gas or vapour / Weight of 1 molecule of hydrogen ……….(i) Now, Molecular Weight = Weight of 1 molecule of gas or vapour / Weight of 1 atom of hydrogen ……….(ii) Dividing (ii) by (i), we get- Molecular Weight / Vapour Density = Weight of 1 molecule of hydrogen / Weight of 1 atom of hydrogen = 2/1 = 2 (because a molecule of hydrogen contains 2 atoms) Therefore, Molecular Weight = 2 x Vapour Density |
(III) It has helped in the determination of weight-volume relationship of gases.
Gram molecular weight (molecular weight expressed in grams- one mole) of any gas occupies a volume of 22.4 litres at N.T.P. or S.T.P. This volume is called Gram Molecular Volume (G.M.V) or Molar Volume. It can be proved with the help of Avogadro’s law as under-
Consider any gas say Y.
| Volume Density of gas, Y = Weight of 22.4 litres of gas Y at S.T.P. / Weight of 22.4 litres of hydrogen at S.T.P. Thus, Weight of 22.4 litres of gas Y at S.T.P. = Weight of 22.4 litres of hydrogen at S.T.P. x Vapour Density of gas, Y = 22.4 x Weight of 1 litre of hydrogen at S.T.P. x Vapour Density of gas, Y = 22.4 x 0.09 x Vapour Density of gas, Y = 2.016 x Vapour Density of gas, Y = Molecular Weight of gas, Y Hence, the molecular weight in grams of any gas, Y, occupies 22.4 litres at S.T.P. |
It may be stated here that one mole (gram molecular weight) of any gas has the same number of molecules, 6.023 x 1023. This number is known as Avogadro’s Number.
(IV) It has helped in establishing molecular formulae of gases.
To illustrate this, let us calculate the molecular formula of ammonia by Avogadro’s law.
According to experimental observation, 1 volume of nitrogen combines with 3 volumes of hydrogen to form 2 volumes of ammonia.
| Thus, 1 volume of nitrogen + 3 volumes of hydrogen ——–> 2 volumes of ammonia or n molecules of nitrogen + 3n molecules of hydrogen ———-> 2n molecules of ammonia or 1 molecule of nitrogen + 3 molecules of hydrogen ———-> 2 molecules of ammonia or 1/2 molecule of nitrogen + 3/2 molecules of hydrogen ———-> 1 molecule of ammonia Since nitrogen and hydrogen are diatomic, we have, 1 atom of nitrogen + 3 atoms of hydrogen ———-> 1 molecule of ammonia |
Therefore, a molecule of ammonia is formed of one atom of nitrogen and three atoms of hydrogen. Hence, the molecular formula of ammonia is NH3.
(V) It has helped in the interpretation of Gay-Lussac’s Law.
For illustration, assume the reaction between ‘a’ molecules of the gas X and ‘b’ molecules of the gas Y, here a and b are simple whole numbers. Let us suppose that 1 cc of each gas contains an equal number of molecules say M under similar conditions of temperature and pressure (Avogadro’s law). Thus, volumes of reacting gases X and Y are a/M x cc and b/M x cc, respectively. These volumes are related to each other as a : b, which is a simple ratio. This is Gay-Lussac’s Law of gaseous volumes.








Comments (No)