Concentration of a Solution:
The concentration of a solution is the amount of solute dissolved in a known amount of solvent or solution. It is used to compare the relative strengths of solutions. The following ways are used to express the concentration of a solution-
(1) Mass Percentage- It is defined as a number of parts by mass of solute present per 100 parts by mass of solution, i.e.,
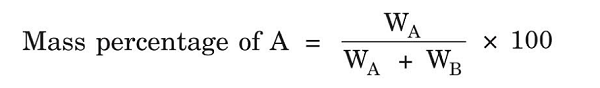
Where WA = Mass of solute, WB = Mass of solvent
Example- 10% of solution of NaCl in H2O means 10 gm of NaCl dissolved in 100 gm of solvent.
(2) Mole Fraction– The mole fraction of any component in the solution is the fraction obtained by dividing the number of moles of that component present in the solution by the total number of moles of the solution. It is denoted by ‘x’.
If nA and nB are the number of moles of a solvent and solute respectively,
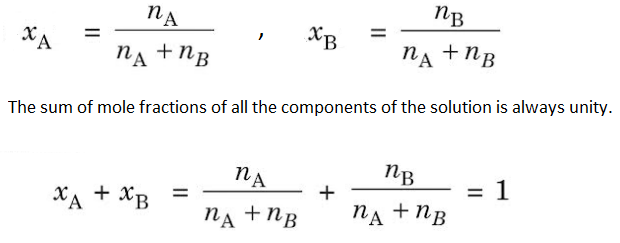
Mole fraction is independent of temperature and has no units. Example- When 40 gm of NaOH is dissolved in 18 gm of H2O, then-
x(NaOH) = (40/40)/[(40/40) + (18/18)] = 1/(1 + 1) = 0.5 [∵ No. of moles = Mass of substance/Molecular Mass].
(3) Molarity- It is defined as the number of moles of solute in grams present in 1 litre of solution. It is denoted by ‘M’, i.e.
| M = Number of moles of solute/Volume of solution in litres Mathematically, M = (a/Molecular Mass) x (1000/V) Where a = mass of solute, V = volume of solution in mL |
Example- A solution of H2SO4 having 4.9 gm of it dissolved in 500 cm3 of solution has Molarity, M = (4.9/98) x (1000/500) = 0.1 moles/litre.
Its units are mole L-1 or Molar. Molarity is one of the common measures of expressing concentration which is frequently used in the laboratory. However, it has one disadvantage. It changes with temperature because of the expansion or contraction of the liquid with temperature.
(4) Molality- Molality of a solution is defined as a number of gram moles of solute dissolved in 1000 grams of solvent. It is denoted by ‘m’.
| Mathematically, m = (Number of moles of solute/Mass of solvent in grams) x 1000 |
If ‘a’ grams of solute is dissolved in ‘b’ grams of solvent,
| m = (a/Molecular Mass) x (1000/b) |
Its units are moles kg-1 or Molal.
Example- A solution of anhydrous Na2CO3 (Molecular Mass = 106) having 1.325 grams of it dissolved in 250 grams of H2O will have morality,
m = (1.325/106) x (1000/250) = 0.05 moles/kg = 0.05 molal
Molality is preferred over Molarity because Molality involves the mass of solute and solvent whereas molarity involves the volume of solution which changes with temperature due to expansion and contraction.
(5) Normality- Normality of a solution is defined as the number of grams equivalent of the solute dissolved per litre of the solvent. It is denoted by ‘N’.
| Mathematically, N = No. of gms equivalent of the solute/Volume of solution in litre |
If ‘a’ gm of solute is present in ‘v’ cm3 of solution.
| N = (a/Eq. mass of solute) x (1000/V) |
Example- A solution of H2SO4 having 0.49 gm of it dissolved in 250 ml of solution will have its normality,
N = (0.49/49) x (1000/250) = 0.04 gm eq./litre or 0.04 Normal
| Equivalent mass = Molecular Mass/(Acidity / Basicity / Valency of element) Equivalent mass of H2SO4 = 98/2 = 49 gm |
Like molarity, the normality of a solution also changes with temperature.
(6) Parts Per Million Parts- It is defined as the mass of solute present in 1 million parts by the mass of the solution. This method is used for very dilute solutions. Thus, for solute ‘A’,
| ppmA = (Mass of A/Mass of Solution) x 106 |
It is used for very-very dilute solutions.
(7) Formality- It is the number of formula masses of solute dissolved per litre of solution. It is represented by ‘F’, i.e.,
| F = No. of formula mass of solute/Volume of solution in litres |
It is used to express the concentration of ionic solutes because ionic compounds don’t exist as such in solution and hence moles cannot be calculated.









Comments (No)