Fusion and Freezing:
When a solid substance is gradually heated, its temperature steadily increases until a temperature is reached at which it starts changing into a liquid state. The process of change of state from solid to liquid is known as fusion or melting.
Once the process of melting has started, the temperature of the substance does not increase although heat is still being supplied to it at the same rate. The temperature of the substance shows practically no rise till the whole mass of the solid is converted into the liquid state. If heating continues even after the whole mass of the solid has been changed into the liquid state, the temperature of the liquid so produced increases steadily. The figure shows a typical heating curve for a solid, in which the quantity of heat added is plotted as the abscissa and temperature as the ordinate. The portion AB of the curve shows heating of the solid, the portion BC represents melting and the portion CD shows further heating of the liquid. From the heating curve, we get the following information:
(1) The solid melts at a definite temperature known as the melting point. The temperature corresponding to the horizontal portion BC is the melting point of the substance.
(2) The substance absorbs a certain amount of heat during melting. This amount of heat is represented by the length of the horizontal portion BC of the heating curve and is known as the heat of fusion.
When heat is removed from a liquid, it cools. If the heat is continuously removed, a stage comes when the substance begins to solidify. The process of change of state from the liquid to the solid state is known as freezing or solidification.
The below figure shows a typical cooling curve that results when heat is continuously removed from the substance at a constant rate.
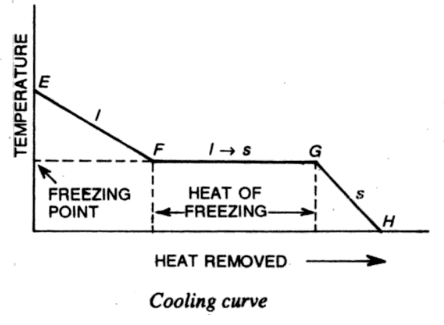
In this curve, the portion EF shows the cooling of the liquid, the portion FG represents the process of solidification and the portion GH corresponds to further cooling of the solid. From the cooling curve, we find that-
(1) The liquid freezes at a definite temperature known as the freezing point. The temperature corresponding to the horizontal portion of the cooling curve gives the freezing point.
(2) The liquid liberates a certain amount of heat during freezing. This amount of heat is represented by the length of the horizontal portion FG of the cooling curve and is known as the heat of freezing.
Experiments show that for a pure and crystalline substance, the melting point and freezing point are the same. However, in the case of non-crystalline substances, these points are not sharp and it is difficult to determine them accurately. The melting point depends on pressure and the presence of impurities.








Comments (No)