Table of Contents
Imperfections or Defects in Solids:
At absolute zero, crystals have a perfectly ordered arrangement that corresponds to minimum energy. With the increase in temperature, the chances of a lattice site being remained unoccupied by the particle increases thereby causing a deviation from the perfectly ordered arrangement. Any deviation from a perfectly ordered arrangement is known as Imperfection or Defect.
Electronic Imperfections:
The electrons in a perfectly covalent or ionic crystal (example- Si or NaCl) are present in fully occupied lowest energy states at 0k. However, at high temperature, some of the electrons may occupy higher energy states which are free to move in the crystals and are responsible for electrical conductivity. The electron-deficient bond formed by the release of an electron is called a Hole which also gives rise to electrical conductivity. In the presence of an electric field, the positive holes move in a direction opposite to that of electrons. Electrons and holes in solids constitute Electronic Imperfections.
Atomic Imperfections:
The defects which arise due to irregularity in the arrangement of atoms or ions are called Atomic Imperfections. When an atomic defect arises due to a missing or misplaced ion, it is known as Point Defect. Points defects in a crystal may be classified into the following three types-
- Stoichiometric Defects.
- Non-Stoichiometric Defects.
- Impurity Defects.
Stoichiometric Defects:
The compounds in which the positive and negative ions are exactly in the ratios indicated by their chemical formula are called Stoichiometric Compounds. The defects that do not disturb the stoichiometry (ratio of a number of positive and negative ions) are called Stoichiometric Point Defects. These are of three types-
- Schottky Defect.
- Frenkel Defect.
- Interstitial Defect.
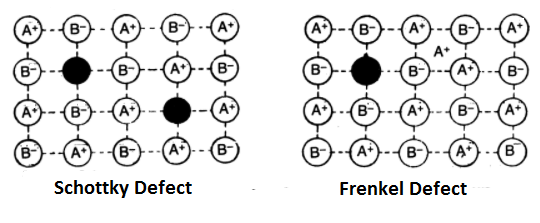
Schottky Defect:
It was discovered in 1930 by a German Scientist Schottky. If in an ionic crystal of the type A+B–, an equal number of cations and anions are missing from their lattice sites leaving behind pair of holes so that the electrical neutrality is maintained, it is called Schottky defect. This defect is more common in highly ionic compounds with a high coordination number. Example- NaCl, KCl, CsCl, KBr etc. As a result of this defect, the number of ions decreases due to which the density of the solid decreases.
Frenkel Defect:
This defect was discovered by Russian Scientist Frenkel in 1926. This defect arises when an ion leaves its correct lattice site and occupies an interstitial site. Frenkel defects are common in ionic compounds which have low coordination number and in which there is a large difference in size between positive and negative ions. Example- AgBr, AgCl, AgI, ZnS etc. Solids having these defects conduct electricity to a small extent. This is because if an ion moves from its lattice site to occupy a hole, it creates a new hole. In this way, a hole may move across the crystal which as a result moves the charge in the opposite direction. This defect causes an increase in the dielectric constant.
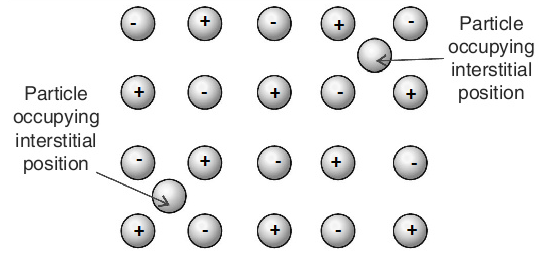
Interstitial Defect:
Interstitial Defect arises when atoms or ions occupy the normally vacant interstitial sites in the crystal. The atoms or ions occupying the interstitial sites are known as Interstitials. The formation of interstitial defects is determined by the size of the interstitial ion. An atom can enter the interstitial site only when it is substantially smaller in size than the parent atom.
Non-Stoichiometric Defects:
The compounds in which the ratio of positive and negative ions present in the compound differs from that required by ideal chemical formulae of a compound are called non-stoichiometric compounds. The defects in these compounds are called non-stoichiometric defects. For example- iron (II) oxide samples contain more oxygen atoms than iron atoms while samples of zinc oxide (ZnO) usually has an excess of zinc atoms than oxygen atoms. However, it may be noted that in each case, the electrical neutrality of the crystal is maintained. These defects are of two types depending upon whether positive ions are in excess or negative ions are in excess. In such defects, there is a change in overall chemical composition. These are also known as metal excess defects and metal deficiency defects, respectively.
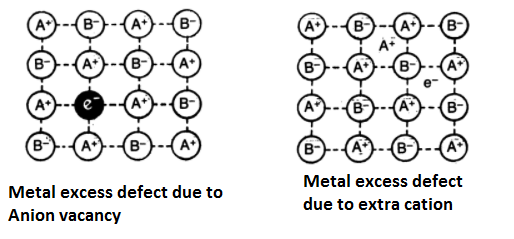
Metal Excess Defects:
In these defects, the positive ions are in excess. These may arise due to the following two ways-
- Anion Vacancies- In this case, negative ions may be missing from their lattice sites leaving holes in which the electrons remain entrapped to maintain electrical neutrality. This is shown in the figure. Evidently, there is an excess of positive (metal) ions although the crystals as a whole is electrically neutral. This type of defect is observed in those crystals which are likely to form Schottky defects. For example- in alkali metal halides, the electrons produced by the ionization of the metal atoms diffuse into the crystal and get trapped at the anion vacancies. The electrons trapped in anion vacancies are referred to as F-centres (from the German word Farbe, meaning colour). These give very interesting properties. For example, the excess of potassium in KCl makes the crystal appear violet, excess fo lithium in LiCl makes the crystal appear pink.
- Excess cations occupying interstitial sites- In this case, there are extra positive ions occupying interstitial sites and the electrons in another interstitial site to maintain electrical neutrality. This is shown in the figure. The defect may be visualized as the loss of non-metal atoms which leave their electrons behind. The excess metal ions occupy interstitial positions. This type of defect is found in crystals that are likely to develop a Frenkel defect. A common example is zinc oxide (ZnO) which loses oxygen reversibly at high temperature and turns yellow in colour. The excess Zn2+ ions are trapped in interstitial sites and an equal number of electrons are trapped in the neighbourhood to balance the electrical charge. These electrons give rise to enhanced electrical conductivity.
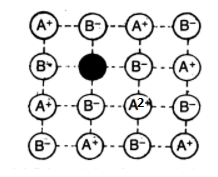
Metal Deficiency Defects:
This defect occurs when the metal shows variable valency i.e. for transition metals. The defect usually occurs due to the missing of a cation from its lattice site and the presence of the cation having a higher charge (example- +2 instead of +1) in the adjacent lattice site. Examples include FeO, FeS and NiO.
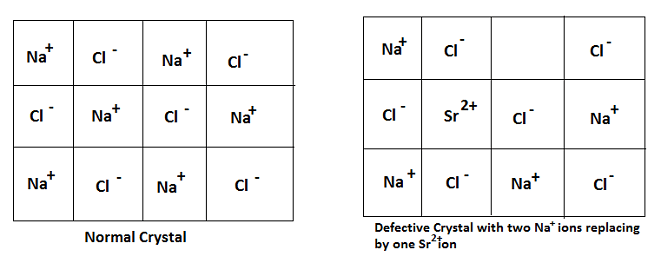
Impurity Defect:
In this type of defect, one or more cations or anions or both are missing from the crystal and these positions are occupied by some other kinds of ions.









Comments (No)