Table of Contents
Kinetic Model and Three States of Matter:
The existence of three states of matter and bulk properties of materials can be explained on the basis of the following two assumptions.
(1) Molecules are in Constant Motion- Besides other factors, molecular motion is related to temperature. The higher the temperature, rapid is the molecular action.
(2) Molecules exert force on one another- When not far, molecules attract each other with a force that falls with the increase in the distance. When the molecules are extremely close to one another, they exert forces of repulsion.
Gaseous State:
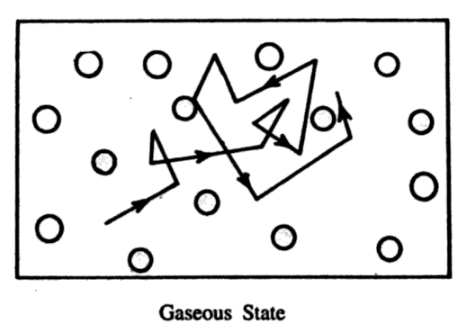
A gas is a substance that has no definite shape, possesses no definite volume, and exerts pressure on the walls of the container.
The molecules of a gas are always in rapid motion. They collide with each other. Due to very rapid motion, they come so close to one another that the forces of repulsion become important and they are forced to rebound after making a collision. A gas exerts pressure on account of the impact of the molecules on the walls of the container.
Liquid State:
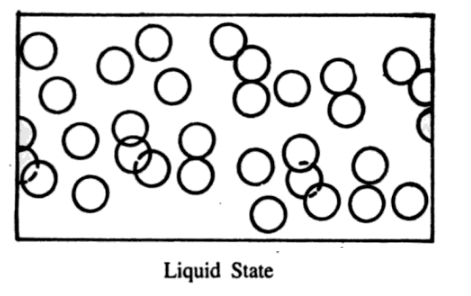
A liquid is a substance that has no definite shape although it possesses a definite volume. Liquids can rise in a capillary tube.
To explain the liquid state, let us imagine a collection of molecules. Suppose the molecules are not in very rapid motion.
Then they will not hit one another with violent forces. There will be little chance of their coming very close and the forces of repulsion would become less significant. Very soon the attractive forces would cause them to collapse together. The average distance between the molecules would reduce. Although liquid molecules can still wander about but there will be little chance of their escaping. As a result, all molecules will be weakly held in a definite volume in a state which we call a liquid state.
Solid State:
A solid is a substance that has a fixed shape and possesses definite volume. It shows the property of elasticity i.e., it resists any change in shape or size.
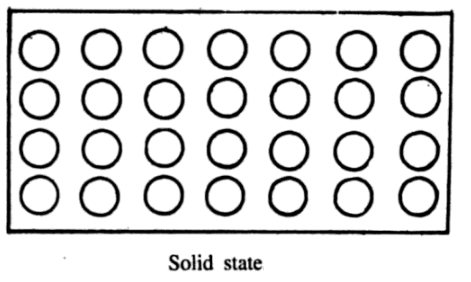
To explain the solid state imagine again a collection of molecules. Suppose the molecular motion is reduced drastically. Now the molecules will not be able to escape from the attractive forces of their neighbors. They can simply vibrate to and fro about their equilibrium positions. The molecules will not wander here and there but form an arrangement that would show mechanical strength and rigidity.








Comments (No)