Table of Contents
Chemical Shift:
The position of signals in the nuclear magnetic resonance (NMR) spectrum helps us to know the type of protons in a molecule i.e., whether these are aromatic, aliphatic, benzylic, etc.
When a molecule is placed in a magnetic field its electrons circulate and thus produce magnetic fields which are called induced magnetic fields.
Now there are two possibilities:
(1) If the induced magnetic fields of electrons oppose the applied magnetic field, then the field felt by the proton is diminished. This process is called “Shielding of Proton”.
For example in acetylene, the induced field of π electrons opposes the applied field at the acetylenic protons, and these protons are thus shielded.
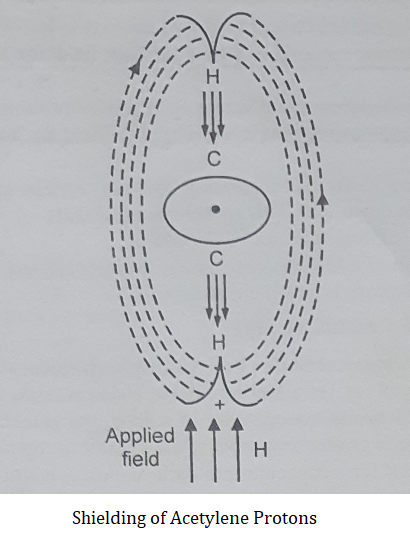
(2) If the induced magnetic field of electrons reinforces the applied magnetic field, then the field felt by the proton is increased. This process is called “Deshielding of Proton”.
For example in benzene, the induced field of circulating π electrons reinforces the applied field at aromatic protons and these protons are thus deshielded.
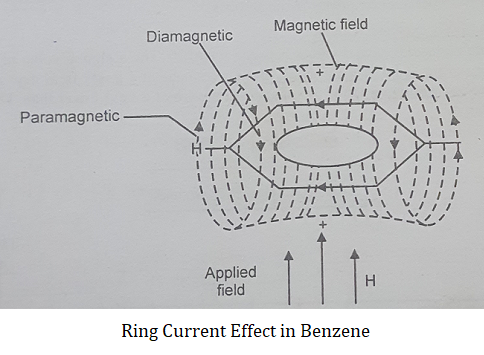
Now in the case of shielding the effective field felt by protons is less than the applied field.
Therefore, shielded protons require a higher applied field for transitions. Thus shielded protons absorb upfield.
Similarly, in the case of deshielding, the effective field felt by protons is more than the applied field. Therefore deshielded protons require a lesser applied field for transitions. Thus deshielded protons absorb downfield.
Therefore shielding shifts the absorption towards upfield and deshielding shifts the absorption towards downfield. This causes a chemical shift.
From above, the chemical shift can be defined as “The shift in the position of NMR absorptions due to shielding and deshielding of protons by an induced magnetic field of electrons is called chemical shift.”
Measurement of Chemical Shift:
For measuring chemical shifts of various protons in a molecule, the signal for Tetra Methyl Silane (TMS) is taken as a reference.
This is because of the low electronegativity of silicon, the shielding of equivalent protons in tetramethylsilane is greater than most of the organic compounds.
Clearly, an NMR signal for a particular proton in a molecule will appear at different field strengths as compared to a signal from (T.M.S.). “This difference in the absorption position of the proton with respect to the TMS signal is also called chemical shift.
Scales for Measuring Chemical Shift: These are two scales.
- δ scale (Delta Scale).
- 𝜏 scale (Tau Scale).
δ scale (Delta Scale)- It is given by the following relation.
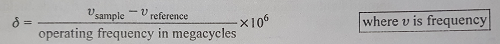
The value of δ is expressed in ppm.
Most of the molecules have δ values between 0 and 10. The assigned value of δ for TMS is zero. In the majority of the organic compounds, protons resonate at a lower field than the protons of TMS. Therefore any proton or set of protons that absorb at a field lower than TMS is given a positive value for δ.
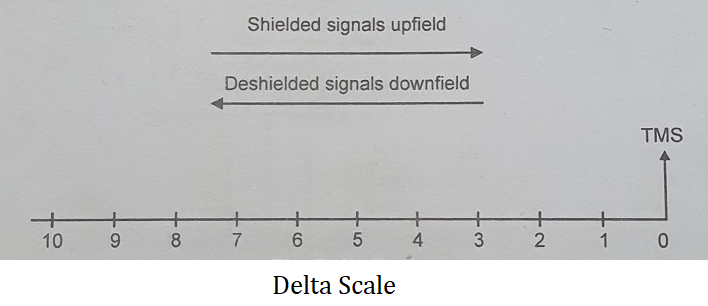
𝜏 scale (Tau Scale)- In this case, TMS is given the value 10 ppm. The δ scale and 𝜏 scale are related by the expression.
| 𝜏 = 10 – δ |
Reasons for Using TMS as a reference compound:
- It is miscible with almost all organic compounds.
- It is highly volatile and is easily removed from the system.
- It does not take part in intermolecular association with the sample.
Factors Affecting Chemical Shift:
There are four factors that affect the chemical shift-
(1) Inductive Effect- If a proton is attached to an electronegative atom or group, then the electronegative atom or group withdraws the electron density around the proton. As we know electrons produce a shielding effect around the protons, therefore if the electron density around the proton is withdrawn by the electronegative atom then the proton will get deshielded and absorbed downfield.

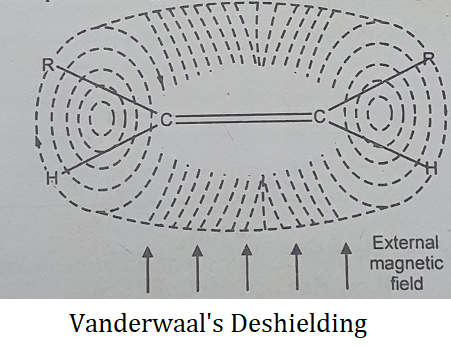
(2) Vander Wall’s Deshielding- In overcrowded molecules, it is possible that some protons may be occupying a sterically hindered position. In such a case, the electron cloud of a bulky group will tend to repel the electron cloud surrounding the proton.
Therefore such a proton will get deshielded and will absorb downfield.
(3) Hydrogen Bonding- It has been observed that hydrogen-bonded compounds absorb downfield, in comparison to the one which does not. This is because the hydrogen-bonded proton being attached to a highly electronegative atom, will have a smaller electron density around it. Therefore the shielding of the proton will be less and the field felt by the proton will be more i.e., absorption will occur downfield.
(4) Anisotropic Effect or Space Effect- π-electrons are more polarizable than sigma-bond electrons. π-electron movement produces strong secondary fields that perturb nearby nuclei. The electron cloud above and below the plane of the ring circulates in reaction to the external field so as to generate an opposing field at the center of the ring and a supporting field at the edge of the ring. This kind of spatial variation is called anisotropy. Regions in which the induced field supports or adds to the external field are said to be deshielded because a slightly weaker external field will bring about resonance for nuclei in such areas. However, regions in which the induced field opposes the external field are termed shielded because an increase in the applied field is needed for resonance.
Solvents used in NMR:
A substance free of protons should be used as a solvent i.e. which does not show absorption of its own in the NMR spectrum. Following solvents are used in NMR spectroscopy i.e. CCl4, CS2, CDCl3, (CCl3)2 C = O, etc.









Comments (No)