Table of Contents
What is ESR Spectroscopy?
ESR Spectroscopy is a branch of absorption spectroscopy in which microwave radiation is absorbed by paramagnetic substances to induce transitions between magnetic levels of electrons with unpaired spins.
It is also called electron paramagnetic resonance (EPR) or electron magnetic resonance (EMR) spectroscopy.
The magnetic splitting is done by applying a static magnetic field.
Types of Species Showing ESR Spectra:
The following types of species show ESR spectra.
(1) Atoms or molecules having an odd number of electrons: for example NO, NO2, O2, etc. They are also called stable paramagnetic substances.
(2) Ions having partly filled inner electron shells. These are usually called free radicals. These may be formed either as intermediates in a chemical reaction or by irradiation of a normal molecule with UV or X-ray.
(3) Molecules that carry angular momentum of electronic origin.
The main interest of Electron Spin Resonance lies in the study of free radicals having unpaired electrons obtained after the hemolytic fission of a covalent bond.
Theory of ESR Spectroscopy:
The free electron behaves as a spinning negatively charged particle (opposite in sign to the proton). By virtue of its charge and spin, an electron acts as a tiny bar magnet and can interact with an external magnetic field.
For an electron spin s = 1/2, the spin angular momentum quantum number will have a value of ms = ±1/2. In the absence of a magnetic field, the two values of m, i.e., +1/2 and -1/2 will give rise to a doubly degenerate spin energy state. If a magnetic field is applied, this degeneracy is removed and this leads to two non-degenerate energy levels. The low energy state will have the spin magnetic moment aligned with the applied field and corresponds to the quantum number ms = -1/2. On the other hand, a high energy state will have the spin magnetic moment as opposed to the applied field and corresponds to the quantum number ms = +1/2.
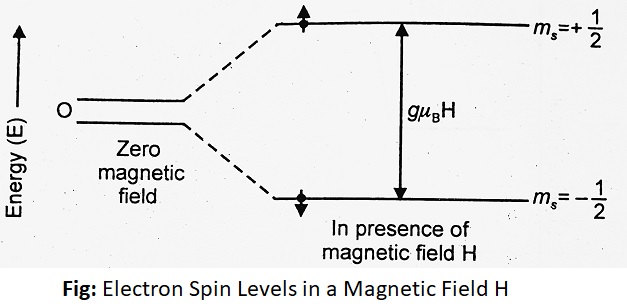
| The Energy associated with each energy state is given by E = µH ……….(i) Where µ = magnetic moment of an e– H = strength of the applied magnetic field But µ = msgβ ……….(ii) Where g = gyromagnetic ratio = 2.0023 ms = spin angular momentum of an electron β = Bohr Magneton = 9.273 x 10-24 JT-1 Putting equation (ii) in equation (i), we get- E = msgβH Where H is the strength of the magnetic field, those pointing toward the direction of an applied magnetic field [spin = -1/2] will decrease in energy by an amount of (1/2) gβH and those pointing opposite to the field [spin = +1/2] will increase in energy by the same amount. The difference in energy between two energy levels is given by ΔE = E2 – E1 As from the above figure, ms = -1/2 for E1 ms = +1/2 for E2 ∴ ΔE = (+1/2) gβH – (-1/2) gβH i.e. ΔE = gβH but ΔE = hν ∴ hν = gβH ∴ ν = gβH/h i.e., So frequency of radiation for this transition ν = gβH/h. |
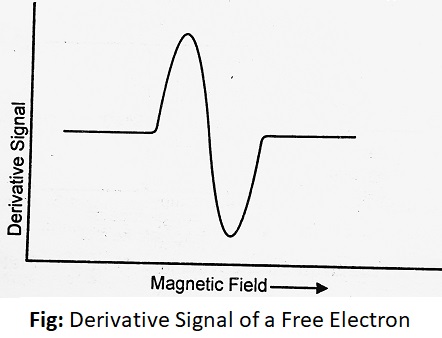
This is called resonance condition which is attained when the frequency ν of incident radiation compares with the frequency corresponding to an energy of separation and a strong absorption occurs. The spectrum of the free electrons would consist of a single peak corresponding to the transition between these two levels.
Applications for ESR Spectroscopy:
When properly interpreted, the ESR spectra give the following useful information about molecules.
(1) The unpaired electron in a species can be located.
(2) The number of lines obtained in the spectrum can decide about the number and type of nuclei present in the neighborhood of the unpaired electron.
(3) The relative intensities in the spectrum not only confirm the number of type of nuclei present in the neighborhood of the unpaired electron but the summation of the intensities can be utilized to evaluate the total number of free electrons in the sample.
(4) The value of g can be measured from the ESR spectrum. This is done by comparing the position of the line with that of the standard substance of known g value.
(5) If the electric field is not spherical, the ESR spectrum is anisotropic i.e., the rotation of the sample shifts the ESR spectrum.








Comments (No)